Working Electrodes
Optimizing your Electrochemical Research
SFtec is dedicated to providing the widest selection of working electrodes for your electrochemical experiments. Our electrodes are characterized by their conventional design, chemical stability, and specific features catering to different detection needs. From conventional electrodes like glassy carbon to specialized solutions for specific analyte detection, SFtec provides a broad spectrum of working electrodes to support your electrochemical research at the right price.
SFtec also offers customized electrode solutions tailored to specific research needs. Contact us if you need assistance selecting the WE that fits better your needs or if you require a customized solution.
Range of Working Electrodes
Working Electrode Technical Notes
Type of Working Electrodes | Features and Applications |
Platinum electrode (PTE) | Conventional electrode, ideal for H2O2 and oxides detection, this electrode is known for its hydrogen adsorptive wave.
|
Gold electrode AUE) | Conventional electrode, splendid for thiols detection due to its absence of hydrogen adsorption wave.
|
Glassy carbon electrode (GCE) |
Offers chemical stability with large over-potentials for oxygen and hydrogen evolutions, making it versatile for numerous tests.
|
Silver electrode (AGE) |
Specialized for cyanide and sulfide detection due to its responsive nature.
|
Carbon paste electrode (CPE) |
Can be mixed with enzymes for creating modified electrodes suitable for specific analytical purposes.
|
Nickel electrode (NIE) |
Chemically modified for amino acids detection, showcasing its adaptability.
|
Palladium electrode (PDE) |
Utilized for studying hydrogen adsorption and desorption processes.
|
Plastic formed carbon electrode (PFCE) |
Features a highly-oriented graphite edge exposed to the surface, offering a cost-effective alternative with characteristics similar to HOPG.
|
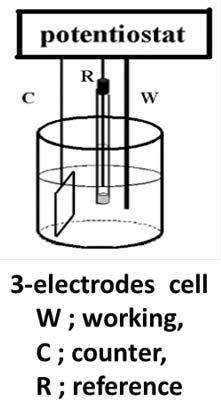
In this guide, we'll share insights on effectively utilizing working electrodes in electrochemical setups. Distinguishing between working and counter electrodes in a two-electrode system can be challenging; they are typically identified as anode or cathode. However, determining the exact potentials of the anode and cathode can be complex, leaving the occurrence of the intended reaction uncertain. It's crucial to assess the redox potential related to the desired reaction by measuring the working electrode's potential.
A clear distinction between working and counter electrodes is achievable when using a potentiostat in a three-electrode setup (as shown in the Figure below). This configuration allows for the precise definition of the working electrode's potential against a reference electrode, enhancing understanding and control over the electrochemical process.
While connecting a platinum wire immersed in the test solution to the potentiostat's reference terminal creates a pseudo-reference electrode, this setup may not offer stable or reproducible potential measurements. Achieving consistent measurements necessitates the use of a reliable reference electrode
Questions often arise about the potential dynamics of the counter electrode. Essentially, the current flow through the working electrode mirrors that of the counter electrode, differing only in being oxidative or reductive. If the counter electrode lacks sufficient depolarizing material relative to the working electrode's reaction, this imbalance could lead to excessive overpotential, potentially exceeding the system's compliance voltage.
Addressing this challenge involves increasing the counter electrode's surface area to reduce current density. This approach, recommending a counter electrode with a larger surface area than the working electrode, is particularly beneficial for applications requiring substantial current flow, such as bulk electrolysis, ensuring smoother operation and minimizing potential issues.
Electrodes are classified into two main types: polarizable and non-polarizable. Polarizable electrodes don't allow Faraday current to flow when their potential changes, making them suitable for use as either working or counter electrodes. On the other hand, non-polarizable electrodes let Faraday current flow once their potential changes, typically serving as reference electrodes.
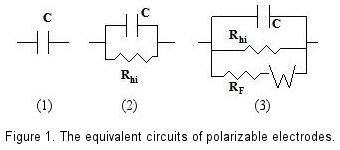
In theoretical models, an ideal polarizable electrode is depicted as a capacitor in an equivalent circuit. Nonetheless, in practice, a minimal current does flow, necessitating a high-value resistor in parallel with the capacitor to accurately represent a real polarizable electrode.
When a redox species is present alongside a polarizable electrode, a redox reaction occurs on the electrode's surface, inducing Faraday current at specific potentials. This situation requires the inclusion of a variable resistance dependent on potential, as well as a Warburg Impedance element to account for the diffusion effects of the species, enhancing the equivalent circuit's complexity. [Fig. 1-(3)]
Figure 1 is a schematic view of above description. In the absence of a redox system, even electrode potential has been changed, the capacitance and high resistance parallel circuit could still be maintained, this variable potential range is so called as potential window.
The concept of a potential window arises in the absence of a redox system, maintaining the capacitance and high resistance circuit even when electrode potential changes. This window is crucial for practical applications, with materials like Platinum, Gold, and Carbon (e.g., glassy carbon) typically offering broad potential windows.
Gold, Platinum and other metal electrodes
Platinum, a common polarizable electrode material, is noted for its stability. However, its use in aqueous solutions demands attention to hydrogen generation and a preceding proton adsorption process. Oxidation at the platinum surface can lead to a reduction current, a phenomenon common to solid metal electrodes. Yet, low target component concentrations may narrow the potential window due to background current interference, although the electrode's theoretical capacitance range primarily determines this window.
This redox paired phenomenon can be founded in most of the solid metal electrode.
When the concentration of the target component is very low, such background current will interfere the measurement and the potential window becomes narrow. Although the electrode potential window is theoretically regulated by the electrode capacitance potential range, there should be no influence for the application if the background current does not interfere to the measurement.
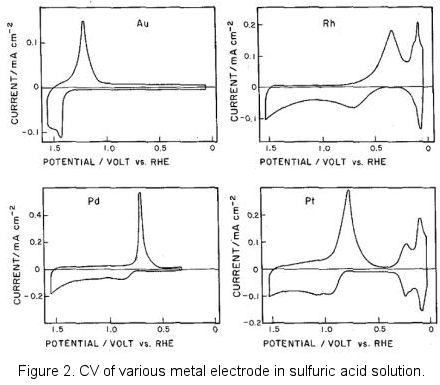
Incidentally, figure 2 above was borrowed from the old literature, negative potential located at the potential axis right side which was known as the classic graphic display method. This reflected the epoch in which the polarography was popularly used in the metallic ions reduction. Today, the IUPAC's convention of displaying positive potential on the right is predominantly used (positive right).
In contrast to aqueous electrolyte solutions, platinum electrodes exhibit a broad potential range in non-proton organic solvents without the occurrence of proton adsorption, desorption, and hydrogen evolution reactions. However, it's crucial to note that in aqueous solutions with a high concentration of chloride ions, platinum can dissolve due to the formation of chloroplatinic acid ions at high oxidation potentials.
Gold, another commonly used electrode material, differs from platinum as it does not exhibit proton adsorption-desorption waves. The over-potential for proton reduction to hydrogen is significantly higher in gold than in platinum, resulting in a wider potential window for gold in the reductive direction in aqueous solutions.
Like platinum, gold can also dissolve in aqueous solutions with high chloride ion concentrations due to the formation of gold chloride acid ions at high oxidation potentials. The gold surface can be easily chemically modified by thiol compounds, making it useful across various research fields.
Carbon, alongside gold and platinum, comes in several forms, including graphite, pyrolytic graphite, highly oriented pyrolytic graphite (HOPG), glassy carbon, and boron-doped diamond electrodes. Among these, glassy carbon is the most commonly used electrode material. Future articles are expected to delve into detailed analyses and chemical modifications of carbon electrode surfaces.
Metallic mercury, liquid at room temperature, can form droplets that fall through a capillary due to gravity, commonly used as a dropping mercury electrode or as a stationary suspension electrode, known as hanging mercury electrode. This classic application in polarography has made mercury electrodes foundational in developing electrochemical reduction analysis methods. Mercury's smooth surface allows for highly reproducible electrode preparation. Its large over-potential for hydrogen ion reduction makes it suitable for detecting various heavy metals (Pb, Tl, In, Cd, Sn, Zn, Ni, Cu, Mn, Fe, Co, Sb, Mg, Ca, Sr, W, etc.). However, due to environmental concerns, the use of mercury, especially in Japan, faces strict regulations.
The working electrodes typically used in electrochemical measurements have been outlined, with the possibility of employing other electrode types for specialized purposes. In corrosion research, for example, iron electrodes are used for Tafel plot polarization measurements, while nickel and nickel-titanium alloy electrodes are chosen for the selective detection of carbohydrates in alkaline solutions. The key takeaway is that various materials can serve as working electrodes in appropriate applications, tailored to specific research objectives.
Graphite, a crystalline form of the carbon element, features a unique structure resembling benzene rings condensed in a plane, forming a hexagonal honeycomb pattern that overlaps in layers known as sp2 carbon. This arrangement is referred to as the basal plane, while the perpendicular surface is termed the edge plane, displaying a layered appearance.
In graphite, there's a distinct anisotropy between the basal and edge planes, particularly in terms of electrical resistance and electrode properties. The basal plane exhibits lower electrical resistance compared to the edge plane, which boasts superior conductivity due to its unique structural orientation. Moreover, the capacitance of the electric double layer on the electrode surface varies, with the basal plane electrode surface showing reduced electric double layer capacitance, highlighting the diverse electrochemical characteristics of graphite.
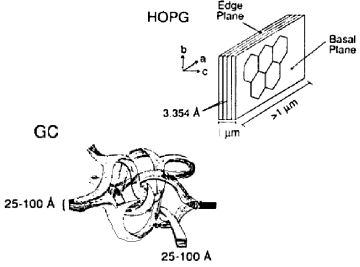
Fig.4-1 The schematics of Graphite and Glassy carbon structure.
- Pyrolytic graphite (PG), produced through the high-temperature decomposition of hydrocarbon gas on a substrate, can achieve enhanced crystalline order under further high-temperature and high-pressure treatment, resulting in highly oriented pyrolytic graphite (HOPG). The electrode's performance is closely linked to its crystalline structure, with the redox peak potential difference (ΔEp) serving as an indicator of surface orderliness. A ΔEp greater than 700 mV suggests a basal plane characteristic of HOPG, contrasting with the smaller ΔEp typically observed in standard graphite electrodes. This difference underscores the slower electron transfer rates at HOPG surfaces.
- Glassy carbon (GC), widely utilized as an electrode material, showcases a complex structure where thin graphite strips interlace, forming what appears to be an amorphous or glassy carbon at the macro level. Despite its microscopic ordered morphology, glassy carbon's overall structure is considered non-crystalline, setting it apart from other carbon allotropes used in electrode materials. Glassy carbon electrodes feature a mix of basal and edge planes, combining the properties of both to offer a dense, impermeable surface ideal for electrochemical applications.
- Additionally, carbon paste electrodes, created by mixing graphite powder with oil into a paste, and carbon fiber electrodes, designed for microelectrode applications, expand the repertoire of carbon-based electrode materials. The introduction of boron into diamond, creating boron-doped diamond electrodes, introduces positive holes in the valence band, enhancing conductivity and offering a wide potential window for specialized electrochemical investigations.
- This exploration into graphite and its derivatives underscores the material's versatility and adaptability as an electrode material, paving the way for advanced electrochemical research and applications.