Reference Electrodes

Extensive array of reference electrodes
Reference electrodes play an important role in the precision and reliability of electrochemical measurements. Discover the ideal reference electrode for your electrochemical measurements, biosensors or HPLC detectors, ensuring accuracy and reliability in your research.
Reference Electrode Selection
The reference of the potential in electrochemical measurement is based on the reference electrode, so it is a very important electrode.
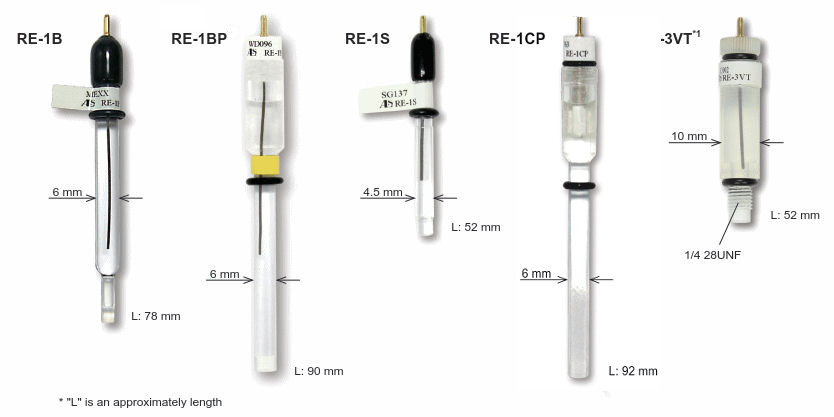
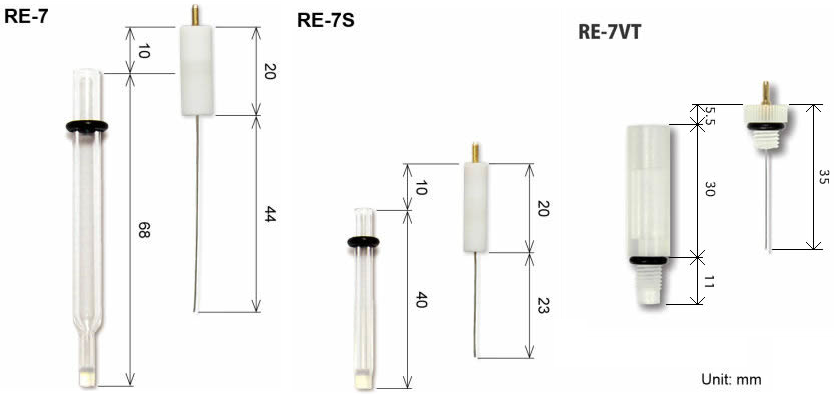
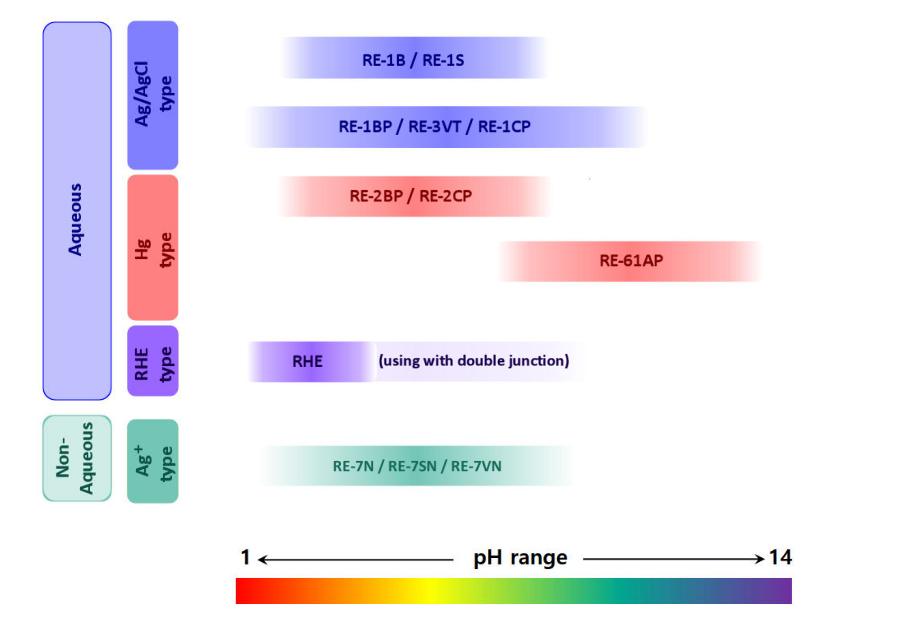
SFTec offers a wide line of reference electrodes, as reference electrode for measurement in aqueous solution, non-aqueous solution, alkaline solution, and reversible hydrogen electrode. Also, the reference electrode has the standard size, which makes possible to fit in all of our accessories.
Furthermore, to complete the line up, ALS screw type electrodes are available, which could be used in: Cross flow cell, Radial flow cell, EQCM flow cell and SEC-Spectroelectrochemical flow cell.
Essential Guide to Reference Electrodes in Electrochemical Research
This comprehensive guide covers the basics of reference electrodes, including types (Ag/AgCl, calomel, Hg-HgO etc.), uses, selection methods, potential calculations, and key considerations, for those new to electrochemical measurements.
Electrochemists rely on reference electrodes to provide a stable potential baseline when controlling and measuring working electrode potentials. This article explains how reference electrodes work, common types, design considerations, and why they enable reproducible electrochemical measurements.
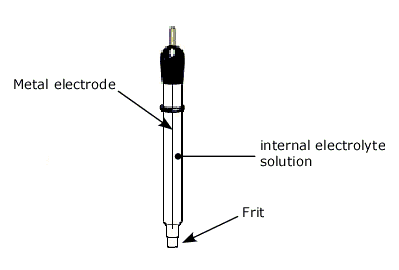
A reference electrode typically contains a metal wire (Pt, Au, Hg, Ag, C, etc.), internal electrolyte solution, and porous junction. It provides a constant half-cell potential, allowing accurate control and measurement of working electrode potentials in electrochemistry experiments.
The key components of a reference electrode are:
- Metal electrode - Platinum, gold, silver, carbon etc. This provides the base potential.
- Internal electrolyte - Contains ions to establish a reversible redox equilibrium at the metal surface.
- Porous Frit - Made of ceramic, Vycor glass etc. Allows ion flow while separating internal and external environments.
Typically a small glass tube (sometimes tubular Teflon for anti-corrosion) is used as the container for these three components. The frit is located at the end of the tube for electric contact with test solution. Requirement of a reference electrode is a long time stability in constant indication of potential. For that, electric current should not be allowed to flow through the reference electrode. Since the current flow may cause a drift of potential, a reference electrode is desired to be connected to a high impedance circuit. Potentiostat has a reference electrode connecting terminal which is high input-impedance terminal. Hence, current flow is strictly limited through a reference electrode.
Specialized mercury-based reference electrodes are also used for some applications. These electrodes are typically housed in a sealed glass or corrosion-resistant Teflon body. The porous frit makes contact with the test solution.
The essential types of reference electrodes, such as the saturated calomel (SCE), silver-silver chloride (Ag/AgCl), and reversible hydrogen electrode (RHE), alongside mercury-based options for specific applications, highlight the versatility and adaptability of reference electrodes to various research needs.
An ideal reference electrode provides a stable potential indefinitely. To achieve this, current flow through the cell must be minimized to prevent potential drift. Reference electrodes are therefore designed to have high impedance.
The internal impedance should also be low for fast potentiostat response times. Physical or chemical processes that increase internal resistance, such as frit clogging, should be prevented.
Ensuring Stability and Reproducibility in Electrochemical Measurements
While connecting a platinum wire immersed in a test solution to a potentiostat's reference terminal may establish electrical contact, dubbed a pseudo-reference electrode, this setup alone does not guarantee the stability or reproducibility of potential measurements. True reproducibility is achieved only by integrating a dependable reference electrode into the experimental setup
The internal impedance of a reference electrode is ideally minimal to prevent any decrease in response rate or potential oscillations due to high impedance in the feedback loop. This underscores the necessity of maintaining the frit at the electrode's end free from deposits that could significantly increase impedance, potentially destabilizing measurements.
Standard hydrogen electrode (SHE) is a primary standard of potential, but it is not necessarily convenient for daily use because of troublesome handling of hydrogen gas. Instead of SHE, as secondary reference electrodes, saturated calomel electrode (SCE), silver-silver chloride electrode (SSCE) and reversible hydrogen electrode (RHE) etc are well known and used.
Why does a Reference Electrode provide a constant potential ?
At the heart of a reference electrode's ability to provide a constant potential is the reversible redox equilibrium at the electrode interface, exemplified by reactions such as the standard hydrogen electrode (SHE), saturated calomel electrode (SCE), and silver-silver chloride electrode (SSCE). These reactions ensure that a reference electrode, maintaining a redox pair in equilibrium, offers an unchangeable external potential, classifying it as a non-polarizable electrode. In contrast, electrodes like platinum, gold, and carbon, whose potentials can be externally adjusted, are termed polarizable electrodes and find use as either working or counter (auxiliary) electrodes.
The key is reversible redox equilibrium at the electrode interface, e.g.:
SHE H2 ⇔ 2H+ + 2e- (0V)
SCE Hg + Cl- ⇔ 1/2Hg2Cl2 + e- (+0.24V)
SSCE Ag + Cl- ⇔ AgCl + e- (+0.20V)
The electrode, which is able to consist of a redox pair in equilibrium is called a reference electrode. The potential of a reference electrode is not able to be changed externally so that it is called non-polarizable electrode. On the contrary, platinum, gold and carbon, of which potential are able to be changed externally are called polarizable electrodes and are used as working or counter (auxiliary) electrodes.
As the relative potentials among the various reference electrodes are fixed and well-known, it is of significant meaning that the comparison between experimental data obtained in even if different places and times is able if the reference electrode used is specified.
Achieving Reference Electrode Equilibrium for Accurate Electrochemistry
Reference electrodes provide the stable potential baseline needed for controlling and measuring working electrodes in electrochemistry experiments. This article explains how common silver/silver chloride reference electrodes work, factors impacting potential stability, proper usage and potential drift checks.
An ideal reference electrode operates at zero current to maintain a fixed potential indefinitely. Reference terminals on potentiostats feature high impedance inputs to prevent current flow through the cell. Without this, fluctuations would shift the reference potential and disrupt working electrode control.
Silver/Silver Chloride Reference Electrodes
The most popular modern reference electrode is silver/silver chloride (SSCE). It offers better stability than old calomel designs without toxic mercury. An equilibrium is established on the silver surface:
The reaction on SSCE is shown as below.
Ag + Cl- ⇔ AgCl + e- (E = +0.20V vs SHE)
Forward reaction is oxidation and reverse is reduction. These reactions are in equilibrium.
Chloride ion (Cl-) comes from internal electrolyte (KCl or NaCl). Ag is silver wire itself. AgCl is thin solid film formed on surface of the silver wire.
It is easy to manufacture Ag/AgCl electrode. It is just anodizing cleaned silver wire in water solution containing chloride ion (KCl or HCl etc.). The film formed on surface of the wire is faint pink and turns to dusky grey with time. Thus obtained wire is put into KCl solution confined in a glass tube with a frit in its end for electric contact with test solution as shown in right Figure. That’s all.
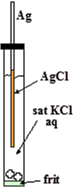
Reference electrode detailed
The electrode potential of above reaction is given as follows.
E = E0Ag+ + (RT/F)ln aAg+ eq.(1)
Substitution of the activity of silver ion with the solubility product constant of AgCl , KsAgCl results eq(1) into following equation (2).
E = E0Ag+ + (RT/F) ln KsAgCl - (RT/F) ln aCl- eq.(2)
Using literature values of E0Ag+ and KsAgCl ( E0Ag+=0.7991 V, and KsAgCl = [Ag+][Cl-] = 1.6×10-10), well-known equation (3) is obtained.
E = 0.222 - (RT/F) ln aCl- eq.(3)
aCl- is the activity of chloride ion in internal solution of the electrode. So, the reference potential of SSCE depends on the activity of chloride ion in addition to temperature. An increase of the activity results in a cathodic shift of the potential. Hence, keeping internal chloride concentration to constant is important to obtain reproducible results.
Reference potential of saturated SSCE(in saturated KCl solution) is 0.197 V at 25°C versus standard hydrogen electrode(SHE). Electric contact of reference electrode with test solution is performed through the frit which permits mutual transfer between the internal solution and the test solution. Hence, dilution of the internal solution and contamination of the test solution provides a possible significant trouble sometimes.
Instead of KCl, sometimes NaCl is chosen as an internal electrolyte. When perchlorate anion is involved in test solution, NaCl is often employed as internal electrolyte because of the low solubility of potassium perchlorate compared with sodium salt. Deposition of potassium perchlorate within the frit may cause serious trouble like large increase of reference electrode impedance.
A main reason of using KCl is that potassium cation and chloride anion have almost identical mobility so that the liquid junction potential at the liquid interface composed of these ions is minimized. While, the liquid junction potential for the case using NaCl as internal electrolyte may become rather considerable. Which one is better choice might depend on a compromise among practical convenience.
Keeping the chloride concentration constant with saturated KCl or NaCl electrolyte is key for reproducibility. Contamination or diffusion can disrupt this equilibrium and alter the reference potential. Preventing material buildup on the frit minimizes these effects.
The mobility ratio between the cation and anion also impacts liquid junction potentials. KCl is often preferred over NaCl since potassium and chloride have nearly equal mobilities. However, NaCl may be used when testing with perchlorate, which has lower solubility with potassium than sodium salts.
Management of reference electrode is important, otherwise resulting in shift of reference potential. Between uses, they should be stored in solution matching the internal electrolyte to prevent contamination. The frit tip must be kept clean - deposits increase impedance which slows potentiostat response.
Check method of Ag/AgCl reference electrode
A simple check compares the membrane potential between an existing and new reference electrode in 3M NaCl or saturated KCl. The expected difference is 0V ± 20mV if both cells are properly functioning. Larger variances indicate deterioration requiring cell replacement.
Reference Potential for difference type of the reference electrode.
NHE (Normal Hydrogen Electrode) | 0 mV |
| SCE (Saturated Calomel Electrode) | 241 mV |
SSCE (Sodium Saturated Calomel Electrode) | 236 mV |
Ag/AgCl (SaturatedNaCl) | 201 mV |
Ag/AgCl (Saturated KCl) | 198 mV |
Hg/HgSO4 (Saturated HgSO4) | 616 mV |
Cu/CuSO4 (Saturated CuSO4) | 300 mV |
The saturated calomel electrode (SCE) served as the dominant reference electrode for decades before environmental concerns over mercury. This article reviews SCE structure, potential-determining reactions, concentration dependencies, temperature effects and usage considerations.
The SCE establishes equilibrium between mercury metal and mercurous chloride:
Hg + Cl- ⇔ 1/2Hg2Cl2 + e- (1)
The potential is described by the Nernst equation with terms for the mercurous chloride redox potential (E0), gas constant (R), temperature (T) and chloride activity (aCl-).
E = E0Hg22+/Hg + RT/2F ln Ks(Hg2Cl2) - RT/F ln aCl- (2)
Early two terms in right side of equation (2) is replaced to E0Hg2Cl2/Hg which is calculated to be 0.273V from the literature values of redox potential
(E0Hg22+/Hg = 0.7960V) and the solubility product constant (Ks(Hg2Cl2)=2×10-18).
Replacing again E0Hg2Cl2/Hg to E0, the equation (3) is obtained, which is familiar formula involving the activity of chloride ion.
E = E0 - (RT/F) ln aCl- (3)
Although a small discrepancy in above calculated value exists, E0 is known as 0.268 V for standard potential at 25°C. The electrode potential depends on the concentration of chloride ion as like silver-silver chloride electrode. It is 0.280 V at 1M KCl and 0.241 V at saturated KCl which is so often called as SCE as abbreviation of Saturated Calomel Electrode.
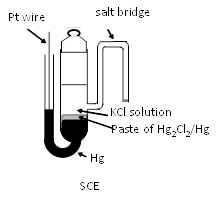
The traditional SCE features a glass body filled with Hg-Hg2Cl2 paste surrounding a KCl reservoir. A porous ceramic frit contacts the test solution. Fragile side-arm salt bridges have been replaced by more convenient ceramic-supported pastes.
The reference potential of SCE is 0.241 V(at 25°C) , because of increased activity of chloride ion in internal solution of saturated KCl (ca. 4.8 M). In addition to the chloride concentration dependence of potential, the temperature dependence of potential is about -0.5 mV per degree. Except for SCE, the reference potentials are 0.280 V and 0.334 V for 1 M KCl and 0.1 M KCl, respectively. The higher the concentration of KCl is, the larger the temperature dependence of the potential is.
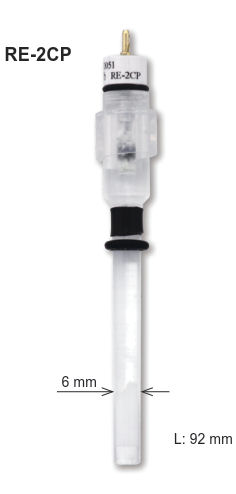
The side arm as salt bridge extended from the body of SCE in above figure is not convenient for daily use because of its fragility. Hence, mercury electrode is placed uppermost and a paste of mercury-mercurous chloride is placed under it and supported by ceramic etc. Such type of SCE electrode is available from BAS Inc. (RE-2BP as shown right Figure).
calomel reference electrode RE-2BP

We often receive the question of "Is SCE usable for experiment in organic solvent?" . Answer is "Yes". However, in the case when you dislike a contamination with water or chloride ion, you are recommended to use a salt bridge, in which both the same organic solvent and electrolyte used in your test solution is contained. Even so, you have to pay attention the fact that a rather large junction potential between SCE and the salt bridge still remains unknown. In addition to that, considering a growing risk of deposition in the frit, it might be better to use a reference electrode for non-aqueous solvent(such as Ag-Ag+ in organic solvent ) or pseudo-reference electrode (such as Pt wire), then refer to the internal standard such as ferrocene.
Usage of pseudo-reference just connecting to the reference terminal of potentiostat is recommendable from the stand point of low impedance.
Despite shifting away from mercury electrodes due to environmental concerns, SCEs remain indispensable in modern electrochemistry due to their reliability. When properly managed, they provide the accuracy needed for quality research.
For electrochemical research that demands avoidance of chloride ion contamination or involves strong alkali or acidic solutions, selecting the right reference electrode is crucial. Traditional Ag/AgCl or calomel electrodes may not always be suitable. In such instances, mercury-mercuric oxide (Hg-HgO) for alkaline environments and mercury-mercurous sulfate electrodes for neutral or acidic contexts provide viable alternatives.
Mercury-Mercuric Oxide Reference Electrode for Alkaline Solutions (Hg-HgO electrode)
Mercury-mercuric oxide electrodes utilize the equilibrium between mercury metal and mercuric oxide/hydroxide layers to provide a stable potential in strongly alkaline solutions up to 14 pH. The cell reaction is:
Hg ⇔ Hg2+ + 2e- (1)
HgO + H2O ⇔ Hg(OH)2 ⇔ Hg2+ + 2OH- (2)
The electrode potential for the reaction (1) is given shown below as equation (3).
E= E0Hg/Hg2+ + (RT/2F) ln aHg2+ (3)
Since the activity of mercuric ion is given by using the solubility product constant as aHg2+ = Ks(Hg(OH)2) /(aOH-)2, equation (3) is replaced to equation (4).
E = E0Hg/Hg2+ + (RT/2F ) ln Ks(Hg(OH)2) - (RT/F ) ln aOH- (4)
As described in the section of silver-silver chloride, the standard potential of the reference electrode utilizing highly insoluble salt depends on significantly its solubility product constant.
Using E0Hg/Hg2+ = 0.8537 and Ks(Hg(OH)2) ≃ 10-25, equation (4) is finally given as below.
E= 0.116 - (RT/F ) ln aOH- (5)
The potential obeys the Nernst equation containing terms for mercuric ion activity and hydroxide concentration. Common internal fill solutions include NaOH, KOH, saturated Ca(OH)2 and Ba(OH)2. The latter two have lower solubility for compatibility with glass containers.
Standard potentials span +0.110V to +0.192V versus the standard hydrogen electrode (SHE), increasing with decreasing hydroxide activity. This allows accurate measurements in environments that deteriorate Ag/AgCl or calomel cells.
Mercury-Mercurous Sulfate Electrodes
Similarly, mercury-mercurous sulfate electrodes provide a reproducible +0.612V potential for neutral or acidic solutions by employing the reaction:
Hg + 1/2SO42- ⇔ 1/2Hg2SO4 + e- (6)
E = E0Hg/Hg22+ + (RT/2F) ln aHg22+ (7)
The equation (7) is replaced by using the relation between the activity of mercurous ion and the solubility product constant Ks(Hg2SO4),
Ks(Hg2SO4) = [Hg22+][SO42-]) as follows.
E = E0Hg/Hg22+ + (RT/2F ) ln Ks(Hg2SO4) - (RT /2F ) ln aSO42- (8)
Putting E0Hg/Hg22+ = 0.7960V(25°C) and Ks(Hg2SO4) ≃ 7×10-7, into equation (8), the final form is shown as below.
E= 0.6125 - (RT/2F ) ln aSO42- (9)
The reference potential is considerably positive compared to that of calomel electrode reflecting rather large solubility of mercurous sulfate. Saturated K2SO4 or 1 M H2SO4 are used as the internal electrolyte. This non-halide design prevents contamination when testing in chlorine-free media. It offers an alternative to calomel cells in low pH environments that damage Ag/AgCl references.
Specialized mercury electrodes expand the working range of electroanalytical measurements. By leveraging different equilibrium reactions, they enable accurate potential control and working electrode measurements in aggressive pH and non-halide media. A line-up of those kinds of electrode are available from ALS Japan as RE-61AP for Hg-HgO electrode and RE-2CP for Hg-Hg2SO4 electrode.