Counter Electrodes
For a system using a three-electrode potentiostat, current is measured when a potential is applied between the working electrode and the reference electrode. Passage of current through an electrical circuit requires electron transfer reaction between the working electrode and the counter electrode.
The main function of the counter electrode is to provide the location of the second electron transfer reaction.
Electrode selection
The main function of the counter electrode is to provide the location of the second electron transfer reaction. Important parameter of the counter electrode is the surface area. It is required (area) large enough to support the current generated for the working electrode.
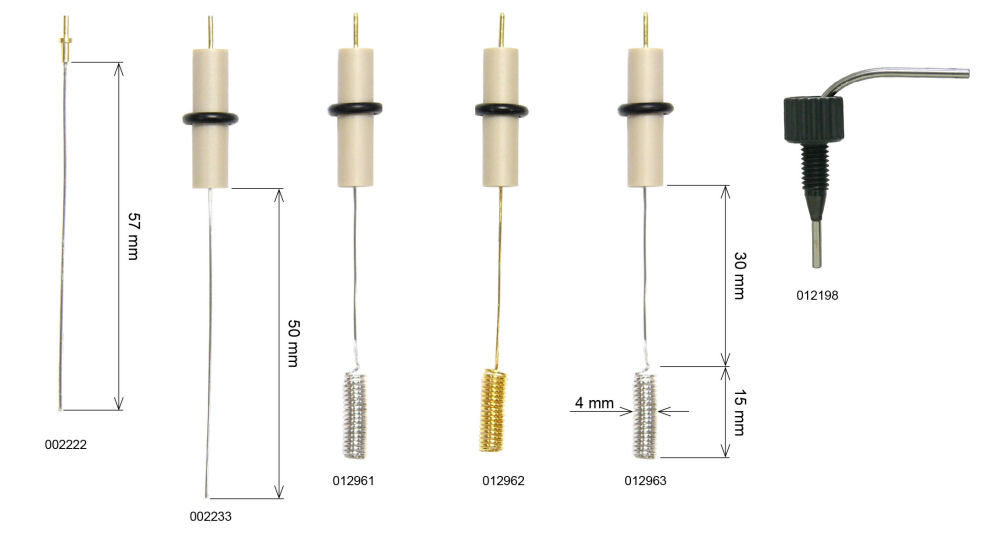
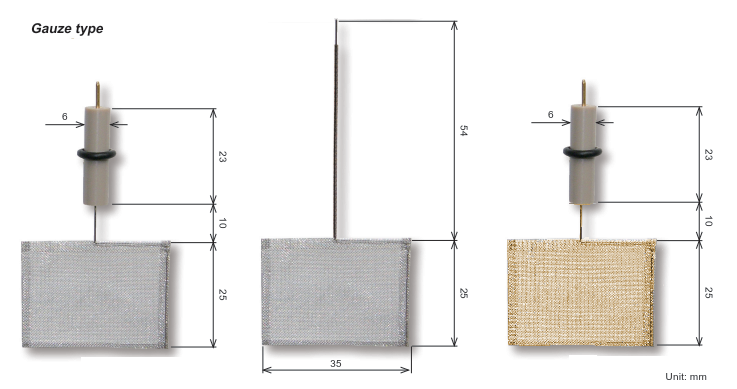
For example, the surface area of the platinum electrode of 5 cm is sufficient to use as an electrode, such as steady-state cyclic voltammetry experiments. However, for generating a high current measurements such as bulk electrolysis, the counter electrode of a larger area is required, as ALS 012961 which the length of platinum is 23 cm. This electrode is used for measurement, such as rotating ring disk.
The cell shape is also an important point. For the electrolysis, to avoid the contamination of the product from the counter electrode, it is arranging separately, isolated in a chamber, from the working electrode. For electrochemical measurements such as cyclic voltammetry, because of the short measurement time, you can ignore the effects of contamination by electrolysis. Therefore, it is not usual the isolation of the counter electrode. In some cases, the separation of the counter electrode in a chamber, increases the resistance between counter electrode and reference electrode, under the influence of (sintered glass) fritz. However, in the case of bulk electrolysis, because of long measurement time, the agitation and separation of the working electrode and counter electrode using a chamber is necessary, to prevent the transportation between two electrodes.
Counter Electrodes Technical Notes
Counter electrode is also called as auxiliary electrode. There is no objection on the former, but somewhat about the latter.
It has the meanings such as preliminary, auxiliary, attached, etc, and it is a word that receives an impression such as something is additional. Of course, that is not the case.
Counter electrode is indispensable for performing electrochemical experiments. Two electrodes are absolutely necessary for current flow and potential measure, which are one working electrode and one counter electrode.
The working electrode is an electrode for conducting a desired reaction (potentiometry is aimed at measuring the potential, so sometimes the working electrode is referred as indicator electrode), then the pairing electrode is the counter electrode.
In the experiment using two electrodes system, it may be difficult to distinguish one from the other. However, in three electrode system using potentiostat, the working electrode and the counter electrode can be clearly distinguished. By using a potentiostat, even if the two-electrode system configuration that counter electrode is connected together with reference electrode at same terminal, the working and counter electrodes are still clearly distinguishable. Therefore, it is desirable to understand working and counter electrodes with relationship to potentiostat.
The current flowing through the working electrode always flows also to the counter electrode. If a reductive (oxidative) reaction occurs at the working electrode, a corresponding coupled oxidative (reductive) reaction occurs at the counter electrode meanwhile. There is no bypass flow path and current only can flow between the counter electrode and the working electrode. Thus, do not forget that the amount of electricity is same in both electrodes.
Assuming a case, which the overvoltage of the counter electrode reaction changes to large, decreasing the counter electrode current density, is the best way. In other words, it is reasonable to make the counter electrode area much larger than that of the working electrode. Otherwise, the overvoltage of the counter electrode becomes large and eventually exceeds the potentiostat allowable output potential range. Especially, an attention should be paid when the electrolysis current increases.
The potential of the working electrode is clearly regulated respecting to the reference electrode potential by the potentiostat, however, the potential of the counter electrode is unknown. Usually it is no much care. One time, there was one detector of LCEC which was able to monitor the potential of the counter electrode. That means there is a person who wants to know what the counter electrode potential is like (make a bad metaphor, just like a coaxing onlookers). The potential of the counter electrode vibrate following the current value of the working electrode. In the counter electrode, if the supporting electrode reaction is insufficient, the overvoltage must also increase. Considering above, that is why the surface area of the counter electrode should be increased as much as possible and the current density should be decreased.
If there is some trepidation that the electrolytic reaction product at the counter electrode may affect the desired electrolysis reaction, it is desirable to place the counter electrode in a compartment separated from the working electrode. Particularly in bulk electrolysis, in order to avoid the working electrode product reverse electrolysis at the counter electrode, it must be installed in a compartment separated by an ion exchange membrane or a ceramic filter.
In the case of impedance measurement, it is necessary to be aware that only characteristics between the reference electrode and the working electrode can be measured using three-electrodes system, whereas, using the two electrodes system, not only the working electrode reaction but also counter electrode reaction will be the characterized.